With the new year beginning, the country is experiencing a surge in respiratory cases of the flu, COVID-19, and RSV. Despite optimism with updated vaccines, challenges like limited RSV vaccine access and low COVID-19 vaccine enthusiasm are hindering efforts.
CDC Data
The Centers for Disease Control and Prevention (CDC) reported an uptick in new hospitalizations at the end of 2023. Seasonal flu activity across the country is currently on the rise and continues to increase in most parts of the country. COVID cases and hospitalizations have also been ticking upward. COVID cases and hospitalizations are also rising. A particular concern is the recent emergence of new Omicron subvariants, that the CDC is tracking called JN.1. In their December 22nd reporting the CDC stated that JN.1 accounted for 39-50% of circulating variants in the United States, which is rapidly becoming the dominant variant across the country. RSV activity remains elevated in many areas of the country.
INFLUENZA
The Advisory Commitee on Immunization Practices (ACIP) recommended routine annual influenza vaccination of all persons aged ≥6 months who do not have contraindications. Vaccination is especially important for these high-risk groups such as:
- Young children
- Pregnant women
- Adults ages 65 and older
- Adults with certain chronic medical conditions, like asthma, heart disease, kidney disease and diabetes
COVID
The ACIP recommendation for all persons 6 months of age and older to receive the 2023-2024 COVID-19 vaccine was officially adopted by the CDC Director on September 12, 2023. CPP is now offering members a discount Moderna’s (Spikevax) and on Pfizer’s (Comirnaty) COVID-19 vaccines.
RSV
There were two immunizations approved by the FDA and recommended by the CDC, AAP and ACOG for protection of infants for the 2023-2024 RSV season:
- Monoclonal Antibody (Sanofi’s Beyfortus) for infants up to 8 months old during their first RSV season
- Vaccine (Pfizer’s Abrysvo) for pregnant women at 32-36 weeks’ gestation during the RSV season
Due to limited supply of Beyfortus, the CDC is strongly encouraging OBGYN practices to recommend and administer Abrysvo to their pregnant patients at 32-36 weeks’ gestation through January 2024.
CPP members can access a discount on Abrysvo doses purchased through Pfizer when enrolled in the CPP/Pfizer-Abrysvo contract.
Vaccination Resources
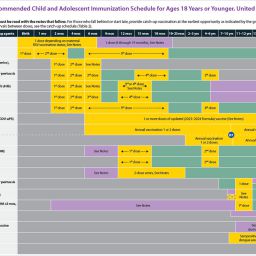
The childhood and adult schedules are helpful resources to share with patients and families to reinforce the importance of staying up to date with their vaccinations. CPP’s purchase ordering guide is another helpful resource to keep your practice on track with ordering routine and seasonal vaccines. The CDC also provides a Healthcare Provider Toolkit to help you educate patients on how to prevent winter respiratory viruses.
Contact CPP
If your practice has any questions regarding flu, COVID or RSV vaccine purchases, please contact CPP at cpp@nationwidechildrens.org. CPP is here to help your practice during this busy respiratory season.